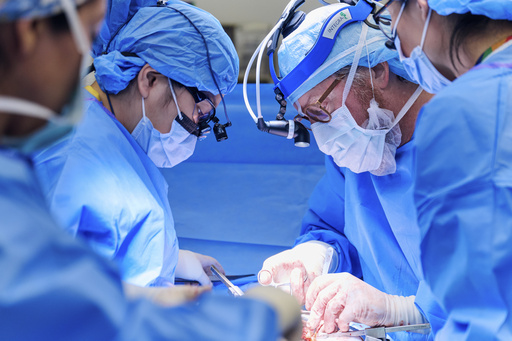
NEW YORK — A woman from Alabama is on the road to recovery after undergoing a pig kidney transplant last month, a significant development in the ongoing exploration of using animal organs to assist human patients. This transplant has liberated her from eight years of being on dialysis, marking her as the fifth individual in the U.S. to receive an organ from genetically modified pigs. Unlike previous patients who passed away shortly after receiving similar xenotransplants, the 53-year-old Towana Looney appears to be doing much better.
“It feels like a fresh start,” Looney shared, describing her experience a month after the procedure. She felt an incredible surge of energy upon receiving the transplanted kidney, exclaiming that the sensation of having a functioning kidney is “unbelievable.” Dr. Robert Montgomery from NYU Langone Health, who headed the groundbreaking operation on November 25, noted that Looney’s successful recovery is a promising indicator as researchers prepare to kick-off formal studies in xenotransplantation planned for next year.
Following the transplant, Looney was reported to be recovering satisfactorily and was released from the hospital just 11 days post-surgery. However, she was readmitted briefly for some adjustments to her medications, with expectations that she can return to her home in Gadsden, Alabama, in three months. If the transplanted kidney does not function properly, she may need to resume dialysis treatments.
Dr. Jayme Locke, who initially performed the surgery and secured FDA approval for the procedure, expressed her excitement in witnessing renewed hope for Looney and her family. With over 100,000 individuals awaiting organ transplants in the United States — primarily needing kidneys — many people die while waiting, and numerous others do not qualify for transplants. As a result, scientists have been genetically modifying pigs to create organs that are more compatible with human biology.
Looney had previously donated a kidney to her mother in 1999, but complications from later pregnancies led to high blood pressure that ultimately damaged her remaining kidney, resulting in its failure. It is rare for living kidney donors to experience such failures, but those who do receive increased priority on the transplant list. Despite this, Looney faced significant hurdles in finding a compatible kidney owing to the development of antibodies that would reject any human organ.
Upon discovering that research on pig kidneys was being conducted at the University of Alabama at Birmingham, Looney expressed her interest in participating in the program. In April 2023, Dr. Locke submitted an emergency application to the FDA for Looney to receive the experimental transplant. Initially, the FDA hesitated, allowing two patients with severe health problems to have the first gene-edited pig kidney transplants in the spring; both patients unfortunately succumbed to complications, leaving Looney undeterred.
When Montgomery surgically implanted the pig kidney, it began functioning well immediately, turning a bright pink and producing urine. Looney remarked that the results are uncertain until there is a practical attempt. The kidney was sourced from a pig that has undergone ten genetic edits, provided by Revivicor, a firm based in Blacksburg, Virginia. Its parent company, United Therapeutics, announced intentions to submit an application to the FDA soon for initiating clinical trials utilizing similar types of organs.
Looney was initially discharged wearing monitoring devices to evaluate her vital signs and was returning to the hospital regularly for follow-up appointments, in addition to the medication readmission. Medical professionals closely monitored her lab results and compared them with previous animal and human studies to identify any early warning signs of potential complications.
Montgomery expressed that many of the observations they are making are unprecedented in the field. Dr. Locke, who has recently begun a role with the federal Health Resources and Services Administration, visited Looney’s hospital room last week, where she was greeted with a hug and gratitude for the perseverance shown throughout this process.
“Thank you for not giving up on me,” Looney said, to which Locke responded with a simple, “Never.”
